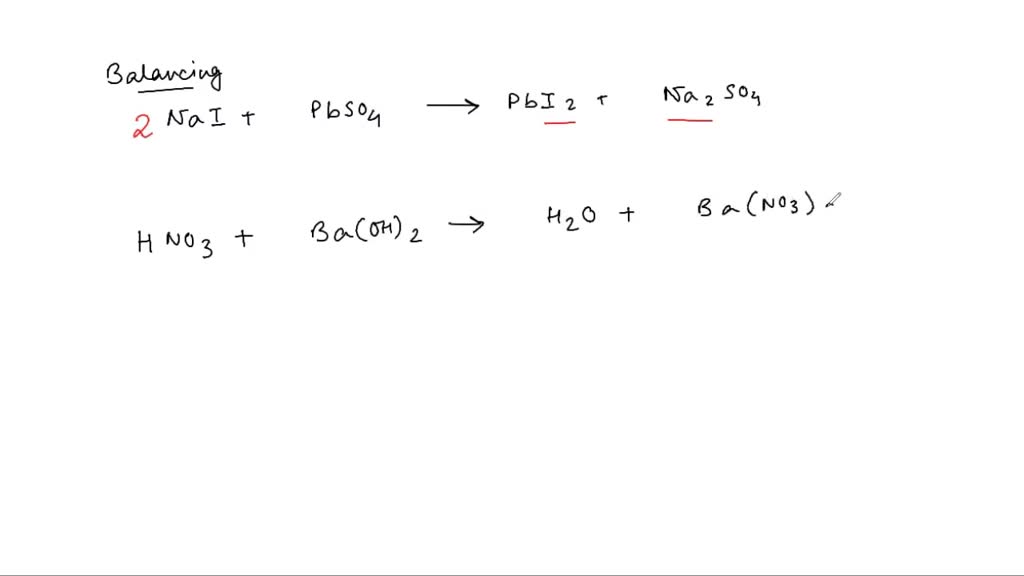
Lead Ii Iodide Balanced Equation . lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. How do you write the balanced equation for this?. It's known for its bright yellow color and low solubility in water, often. The balanced equation will be calculated along with the. lead (ii) iodide is an ionic compound with the chemical formula pbi2. this is the overall balanced chemical equation for the reaction, showing the reactants and products in their undissociated form. the most complex substance is lead (ii) chloride. this page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. enter an equation of an ionic chemical equation and press the balance button. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2.
from www.numerade.com
the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. lead (ii) iodide is an ionic compound with the chemical formula pbi2. \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. the most complex substance is lead (ii) chloride. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. enter an equation of an ionic chemical equation and press the balance button. this is the overall balanced chemical equation for the reaction, showing the reactants and products in their undissociated form. How do you write the balanced equation for this?. The balanced equation will be calculated along with the. this page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution.
SOLVED What are the coefficients for the following reaction when it is
Lead Ii Iodide Balanced Equation this is the overall balanced chemical equation for the reaction, showing the reactants and products in their undissociated form. lead (ii) iodide is an ionic compound with the chemical formula pbi2. this is the overall balanced chemical equation for the reaction, showing the reactants and products in their undissociated form. enter an equation of an ionic chemical equation and press the balance button. \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. the most complex substance is lead (ii) chloride. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. this page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. It's known for its bright yellow color and low solubility in water, often. The balanced equation will be calculated along with the. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. How do you write the balanced equation for this?.
From www.youtube.com
007 Potassium Iodide + Lead (II) Nitrate = Lead (II) Iodide Lead Ii Iodide Balanced Equation the most complex substance is lead (ii) chloride. It's known for its bright yellow color and low solubility in water, often. lead (ii) iodide is an ionic compound with the chemical formula pbi2. How do you write the balanced equation for this?. The balanced equation will be calculated along with the. the chemical equation described in section. Lead Ii Iodide Balanced Equation.
From martlabpro.com
Lead Nitrate And Potassium Iodide Balanced Equation An Overview Lead Ii Iodide Balanced Equation The balanced equation will be calculated along with the. How do you write the balanced equation for this?. this page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. It's known for its bright yellow color and. Lead Ii Iodide Balanced Equation.
From www.showme.com
Ksp of lead II iodide Science, Chemistry, Balancing Equations Lead Ii Iodide Balanced Equation the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. The balanced equation will be calculated along with the. lead (ii) iodide is an ionic compound with the chemical formula pbi2. enter an equation of an ionic chemical equation and press the balance button. How do. Lead Ii Iodide Balanced Equation.
From www.numerade.com
SOLVED Write the correct net ionic equation for the reaction of Lead Ii Iodide Balanced Equation the most complex substance is lead (ii) chloride. lead (ii) iodide is an ionic compound with the chemical formula pbi2. this is the overall balanced chemical equation for the reaction, showing the reactants and products in their undissociated form. this page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution.. Lead Ii Iodide Balanced Equation.
From byjus.com
Write word equation and balanced chemical equation for the following Lead Ii Iodide Balanced Equation The balanced equation will be calculated along with the. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. this is the overall balanced chemical equation for the reaction, showing the reactants and products in their undissociated form. this page discusses the. Lead Ii Iodide Balanced Equation.
From webapi.bu.edu
Lead iodide formula. Lead Iodide Structure, Preparations and Lead Ii Iodide Balanced Equation lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. the most complex substance is lead (ii) chloride. How do you write the balanced equation for this?. It's known for its bright yellow color and low solubility in water, often. the chemical equation described in section 4.1 is balanced, meaning that equal. Lead Ii Iodide Balanced Equation.
From martlabpro.com
Lead Nitrate And Potassium Iodide Balanced Equation An Overview Lead Ii Iodide Balanced Equation It's known for its bright yellow color and low solubility in water, often. enter an equation of an ionic chemical equation and press the balance button. this page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. How do you write the balanced equation for this?. this is the overall balanced. Lead Ii Iodide Balanced Equation.
From exoncvmev.blob.core.windows.net
Lead Ii Nitrate And Sodium Chloride Balanced Equation at Janice Timmons Lead Ii Iodide Balanced Equation \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. How do you write the balanced equation for this?. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. The balanced equation will be calculated along with the. this is the overall balanced chemical equation for the reaction, showing the reactants and products. Lead Ii Iodide Balanced Equation.
From www.youtube.com
How to write the formula for lead (II) iodide YouTube Lead Ii Iodide Balanced Equation \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. the most complex substance is lead (ii) chloride. How do you write the balanced equation for this?. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. this is the overall balanced chemical equation for the. Lead Ii Iodide Balanced Equation.
From www.toppr.com
Write the balanced chemical equation the following reactions & identify Lead Ii Iodide Balanced Equation lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. It's known for its bright yellow color and low solubility in water, often. The balanced equation will be calculated along with the. this page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. lead (ii) iodide. Lead Ii Iodide Balanced Equation.
From slideplayer.com
Precipitation Reactions ppt download Lead Ii Iodide Balanced Equation this is the overall balanced chemical equation for the reaction, showing the reactants and products in their undissociated form. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. lead (ii) nitrate reacts with potassium iodide. Lead Ii Iodide Balanced Equation.
From www.shutterstock.com
Leadii Iodide Lead Iodide Formula Pbi2 Stock Illustration 729111571 Lead Ii Iodide Balanced Equation this is the overall balanced chemical equation for the reaction, showing the reactants and products in their undissociated form. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. How do you write the balanced equation for this?. It's known for its bright yellow color and low solubility in water, often. lead. Lead Ii Iodide Balanced Equation.
From www.numerade.com
SOLVED If you dissolve lead (II) nitrate and potassium iodide in water Lead Ii Iodide Balanced Equation the most complex substance is lead (ii) chloride. It's known for its bright yellow color and low solubility in water, often. The balanced equation will be calculated along with the. this page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. the chemical equation described in section 4.1 is balanced, meaning. Lead Ii Iodide Balanced Equation.
From www.numerade.com
SOLVED Based on solubility rules, lead (II) iodide (PbI2) is an Lead Ii Iodide Balanced Equation lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. lead (ii) iodide is an ionic compound with the chemical formula pbi2. this page discusses the precipitation of insoluble lead. Lead Ii Iodide Balanced Equation.
From phonegallery1.blogspot.com
Check Zinc Lead Ii Acetate Balanced Equation Updated 2021 Phone Gallery Lead Ii Iodide Balanced Equation this page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. How do you write the balanced equation for this?. \[\ce{pb(no3)2(aq) + nacl(aq) → nano3(aq) + pbcl2(s)} \nonumber \] 2. this is the overall balanced chemical equation for the reaction, showing the reactants and products in their undissociated form. the most. Lead Ii Iodide Balanced Equation.
From www.coursehero.com
[Solved] A chemist mixes aqueous potassium iodide with lead (II Lead Ii Iodide Balanced Equation lead (ii) iodide is an ionic compound with the chemical formula pbi2. How do you write the balanced equation for this?. enter an equation of an ionic chemical equation and press the balance button. lead (ii) nitrate reacts with potassium iodide to form lead (ii) iodide and potassium nitrate. It's known for its bright yellow color and. Lead Ii Iodide Balanced Equation.
From www.numerade.com
SOLVED What are the coefficients for the following reaction when it is Lead Ii Iodide Balanced Equation The balanced equation will be calculated along with the. lead (ii) iodide is an ionic compound with the chemical formula pbi2. the most complex substance is lead (ii) chloride. It's known for its bright yellow color and low solubility in water, often. this page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions. Lead Ii Iodide Balanced Equation.
From www.slideserve.com
PPT Reactions in Aqueous Solution PowerPoint Presentation, free Lead Ii Iodide Balanced Equation this is the overall balanced chemical equation for the reaction, showing the reactants and products in their undissociated form. How do you write the balanced equation for this?. lead (ii) iodide is an ionic compound with the chemical formula pbi2. this page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution.. Lead Ii Iodide Balanced Equation.